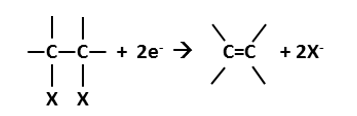Difference between revisions of "Biodegradation - Reductive Processes"
m (1 revision imported) |
|||
| (11 intermediate revisions by 2 users not shown) | |||
| Line 4: | Line 4: | ||
'''Related Article(s)''': | '''Related Article(s)''': | ||
| + | |||
*[[Bioremediation - Anaerobic]] | *[[Bioremediation - Anaerobic]] | ||
*[[Chemical Reduction (In Situ - ISCR)]] | *[[Chemical Reduction (In Situ - ISCR)]] | ||
| + | *[[Chlorinated Solvents]] | ||
| + | *[[REMChlor - MD]] | ||
| − | ''' | + | '''Contributor(s):''' [[Dr. David L. Freedman]] |
'''Key Resource(s)''': | '''Key Resource(s)''': | ||
| − | *[https://doi.org/10.1002/jctb.1567 Enhanced Anaerobic Bioremediation of Chlorinated Solvents: Environmental Factors Influencing Microbial Activity and Their Relevance under Field Conditions]<ref name= "Aulenta2006">Aulenta, F., Majone, M. and Tandoi, V., 2006. Enhanced anaerobic bioremediation of chlorinated solvents: environmental factors influencing microbial activity and their relevance under field conditions. Journal of Chemical Technology and Biotechnology, 81(9), 1463-1474. [https://doi.org/10.1002/jctb.1567 doi: 10.1002/jctb.1567]</ref>. | + | |
| − | *[https://doi.org/10.1007/978-3-662-49875-0 Organohalide Respiring Bacteria]<ref name= "Adrian2016">Adrian, L. and Löffler, F., 2016. Organohalide Respiring Bacteria. 495 pgs. ISBN: 978-3-662-49873-6. [https://doi.org/10.1007/978-3-662-49875-0 doi: 10.1007/978-3-662-49875-0]</ref> | + | *[https://doi.org/10.1002/jctb.1567 Enhanced Anaerobic Bioremediation of Chlorinated Solvents: Environmental Factors Influencing Microbial Activity and Their Relevance under Field Conditions]<ref name="Aulenta2006">Aulenta, F., Majone, M. and Tandoi, V., 2006. Enhanced anaerobic bioremediation of chlorinated solvents: environmental factors influencing microbial activity and their relevance under field conditions. Journal of Chemical Technology and Biotechnology, 81(9), 1463-1474. [https://doi.org/10.1002/jctb.1567 doi: 10.1002/jctb.1567]</ref>. |
| + | *[https://doi.org/10.1007/978-3-662-49875-0 Organohalide Respiring Bacteria]<ref name="Adrian2016">Adrian, L. and Löffler, F., 2016. Organohalide Respiring Bacteria. 495 pgs. ISBN: 978-3-662-49873-6. [https://doi.org/10.1007/978-3-662-49875-0 doi: 10.1007/978-3-662-49875-0]</ref> | ||
==Introduction== | ==Introduction== | ||
| Line 26: | Line 30: | ||
==Bioremediation== | ==Bioremediation== | ||
| − | As a general rule, bioremediation is a lower cost approach to treatment of halogenated solvents than competing processes based on physical or chemical techniques (e.g., [[Chemical Oxidation (In Situ - ISCO) | chemical oxidation]]). The discovery that microbes are capable of replacing the chlorines on tetrachloroethene (PCE) and TCE, the two most frequently encountered organic contaminants at hazardous waste sites, opened the door to development of current bioremediation strategies. Notably, there was a pause in interest when it was initially believed that the dechlorination process stopped at vinyl chloride (VC), which is more toxic than PCE, TCE, and dichloroethene (DCE) isomers. It was subsequently determined that microbial reduction of VC to ethene occurs<ref>Freedman, D.L. and Gossett, J.M., 1989. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Applied and Environmental Microbiology, 55(9), 2144-2151. [ | + | As a general rule, bioremediation is a lower cost approach to treatment of halogenated solvents than competing processes based on physical or chemical techniques (e.g., [[Chemical Oxidation (In Situ - ISCO) | chemical oxidation]]). The discovery that microbes are capable of replacing the chlorines on tetrachloroethene (PCE) and TCE, the two most frequently encountered organic contaminants at hazardous waste sites, opened the door to development of current bioremediation strategies. Notably, there was a pause in interest when it was initially believed that the dechlorination process stopped at vinyl chloride (VC), which is more toxic than PCE, TCE, and dichloroethene (DCE) isomers. It was subsequently determined that microbial reduction of VC to ethene occurs<ref>Freedman, D.L. and Gossett, J.M., 1989. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Applied and Environmental Microbiology, 55(9), 2144-2151. [//www.enviro.wiki/images/9/96/Freedman-1989-Biological_reductive_dechlorination.pdf Report pdf]</ref>. Ethene is an acceptable endpoint since it poses no human health risks at the concentrations typically found in groundwater. |
Although humans are responsible for a large influx of halogenated organics into the environment as a consequence of improper handling and disposal practices, it has also come to light that there are natural sources of halogenated organic compounds; nearly 5,000 have been catalogued to date<ref>Gribble, G. W., 2010. Naturally Occurring Organohalogen Compounds - A Comprehensive Update. SpringerWien: New York. [https://doi.org/10.1007/978-3-211-99323-1 doi: 10.1007/978-3-211-99323-1]</ref>. For example, marine algae produce chloromethane as part of a chemical defense system to dissuade predation. Consequently, it is not too surprising that natural processes exist to break down halogenated organic compounds, and those processes have been harnessed to help clean up the excessive amounts released to the environment as a consequence of human activity. | Although humans are responsible for a large influx of halogenated organics into the environment as a consequence of improper handling and disposal practices, it has also come to light that there are natural sources of halogenated organic compounds; nearly 5,000 have been catalogued to date<ref>Gribble, G. W., 2010. Naturally Occurring Organohalogen Compounds - A Comprehensive Update. SpringerWien: New York. [https://doi.org/10.1007/978-3-211-99323-1 doi: 10.1007/978-3-211-99323-1]</ref>. For example, marine algae produce chloromethane as part of a chemical defense system to dissuade predation. Consequently, it is not too surprising that natural processes exist to break down halogenated organic compounds, and those processes have been harnessed to help clean up the excessive amounts released to the environment as a consequence of human activity. | ||
| − | There are several types of reaction pathways that involve reduction and dehalogenation, including [[wikipedia:Hydrogenolysis |hydrogenolysis]] and dihaloelimination<ref>Vogel, T.M., Criddle, C.S. and McCarty, P.L., 1987. ES&T critical reviews: transformations of halogenated aliphatic compounds. Environmental Science & Technology, 21(8), 722-736. [http://dx.doi.org/10.1021/es00162a001 doi:10.1021/es00162a001]</ref>. Here, we detail each of these pathways and the conditions under which each is likely to be prevalent. It should be noted that many of the reactions are also possible via abiotic processes (e.g., via reaction with [[Zerovalent Iron (ZVI | + | There are several types of reaction pathways that involve reduction and dehalogenation, including [[wikipedia:Hydrogenolysis |hydrogenolysis]] and dihaloelimination<ref>Vogel, T.M., Criddle, C.S. and McCarty, P.L., 1987. ES&T critical reviews: transformations of halogenated aliphatic compounds. Environmental Science & Technology, 21(8), 722-736. [http://dx.doi.org/10.1021/es00162a001 doi:10.1021/es00162a001]</ref>. Here, we detail each of these pathways and the conditions under which each is likely to be prevalent. It should be noted that many of the reactions are also possible via abiotic processes (e.g., via reaction with [[Zerovalent Iron (ZVI) (Chemical Reduction - ISCR) |zerovalent iron (ZVI)]])<ref>Arnold, W.A. and Roberts, A.L., 2000. Pathways and kinetics of chlorinated ethylene and chlorinated acetylene reaction with Fe(0) particles. Environmental Science & Technology, 34(9), 1794-1805. [http://dx.doi.org/10.1021/es990884q doi:10.1021/es990884q]</ref>. The focus of this article is on biotic reductive processes. |
==Hydrogenolysis== | ==Hydrogenolysis== | ||
| Line 69: | Line 73: | ||
Hydrogenolysis of brominated organic compounds has been documented. Examples include reduction of 1,2-dibromoethane (ethylene dibromide) to bromoethane and reduction of polybrominated diphenyl ethers. Likewise, defluorination also occurs. For example, reduction of vinyl fluoride to ethene has been reported. Nevertheless, reductive defluorination is characterized by slow rates, if it occurs at all. This is consistent with the general expectation that when the rate-limiting step for a reaction is cleavage of the carbon halogen bond, the order of reactivity is: | Hydrogenolysis of brominated organic compounds has been documented. Examples include reduction of 1,2-dibromoethane (ethylene dibromide) to bromoethane and reduction of polybrominated diphenyl ethers. Likewise, defluorination also occurs. For example, reduction of vinyl fluoride to ethene has been reported. Nevertheless, reductive defluorination is characterized by slow rates, if it occurs at all. This is consistent with the general expectation that when the rate-limiting step for a reaction is cleavage of the carbon halogen bond, the order of reactivity is: | ||
| − | <div align= "center">C—I > C—Br > C—Cl > C—F<ref>Wackett, L.P., Logan, M.S., Blocki, F.A. and Bao-Li, C., 1992. A mechanistic perspective on bacterial metabolism of chlorinated methanes. Biodegradation, 3(1), 19-36. [https://doi.org/10.1007/bf00189633 doi: 10.1007/BF00189633]</ref></div> | + | <div align="center">C—I > C—Br > C—Cl > C—F<ref>Wackett, L.P., Logan, M.S., Blocki, F.A. and Bao-Li, C., 1992. A mechanistic perspective on bacterial metabolism of chlorinated methanes. Biodegradation, 3(1), 19-36. [https://doi.org/10.1007/bf00189633 doi: 10.1007/BF00189633]</ref></div> |
For example, hydrogenolysis of trichlorofluoromethane (CFC-11) typically results in accumulation of dichloro- and chlorofluoro-methane; further hydrogenolysis occurs at a much slower rate, if at all. Microbial reductive defluorination of perflurooctanoic acid (PFOA; used in the manufacture of Teflon) has not been reported to any appreciable extent<ref>Liou, J.C., Szostek, B., DeRito, C.M. and Madsen, E.L., 2010. Investigating the biodegradability of perfluorooctanoic acid. Chemosphere, 80(2), 176-183. [http://dx.doi.org/10.1016/j.chemosphere.2010.03.009 doi: 10.1016/j.chemosphere.2010.03.009]</ref>. | For example, hydrogenolysis of trichlorofluoromethane (CFC-11) typically results in accumulation of dichloro- and chlorofluoro-methane; further hydrogenolysis occurs at a much slower rate, if at all. Microbial reductive defluorination of perflurooctanoic acid (PFOA; used in the manufacture of Teflon) has not been reported to any appreciable extent<ref>Liou, J.C., Szostek, B., DeRito, C.M. and Madsen, E.L., 2010. Investigating the biodegradability of perfluorooctanoic acid. Chemosphere, 80(2), 176-183. [http://dx.doi.org/10.1016/j.chemosphere.2010.03.009 doi: 10.1016/j.chemosphere.2010.03.009]</ref>. | ||
| Line 85: | Line 89: | ||
==Organohalide Respiration== | ==Organohalide Respiration== | ||
| − | A variety of microbes have developed pathways to conserve the energy made available when breaking carbon-halogen bonds via reduction (i.e., hydrogenolysis and dihaloelimination), by using the halogenated organic compounds as terminal electron acceptors<ref name= "Adrian2016"/>. This has led to the notion that certain microbes are capable of “breathing” halogenated organics, analogous to respiration involving oxygen as the electron acceptor<ref name = "McCarty1997">McCarty, P.L., 1997. Breathing with chlorinated solvents. Science, 276(5318), 1521-1522. [https://doi.org/10.1126/science.276.5318.1521 doi: 10.1126/science.276.5318.1521]</ref>. When halogenated organic compounds serve as terminal electron acceptors, the process is referred to as organohalide respiration. The discovery of this process adds to the extensive list of terminal electron acceptors that microbes are capable of exploiting. Notably, a few types of microbes are obligate halorespirers, meaning the only known terminal electron acceptors for the cells are halogenated organic compounds. Other types of microbes are facultative with respect to their use of halogenated organics as terminal electron acceptors. | + | A variety of microbes have developed pathways to conserve the energy made available when breaking carbon-halogen bonds via reduction (i.e., hydrogenolysis and dihaloelimination), by using the halogenated organic compounds as terminal electron acceptors<ref name="Adrian2016" />. This has led to the notion that certain microbes are capable of “breathing” halogenated organics, analogous to respiration involving oxygen as the electron acceptor<ref name="McCarty1997">McCarty, P.L., 1997. Breathing with chlorinated solvents. Science, 276(5318), 1521-1522. [https://doi.org/10.1126/science.276.5318.1521 doi: 10.1126/science.276.5318.1521]</ref>. When halogenated organic compounds serve as terminal electron acceptors, the process is referred to as organohalide respiration. The discovery of this process adds to the extensive list of terminal electron acceptors that microbes are capable of exploiting. Notably, a few types of microbes are obligate halorespirers, meaning the only known terminal electron acceptors for the cells are halogenated organic compounds. Other types of microbes are facultative with respect to their use of halogenated organics as terminal electron acceptors. |
| − | Under some circumstances, microbes carry out reductive dehalogenation but are unable to conserve energy from the process. For example, several strains of microbes are able to use PCE, TCE and ''cis''-DCE as terminal electron acceptors, whereas reduction of VC to ethene is not a growth-linked respiratory process<ref>Maymó-Gatell, X., Anguish, T. and Zinder, S.H., 1999. Reductive dechlorination of chlorinated ethenes and 1, 2-dichloroethane by ''Dehalococcoides ethenogenes'' 195. Applied and Environmental Microbiology, 65(7), 3108-3113. [ | + | Under some circumstances, microbes carry out reductive dehalogenation but are unable to conserve energy from the process. For example, several strains of microbes are able to use PCE, TCE and ''cis''-DCE as terminal electron acceptors, whereas reduction of VC to ethene is not a growth-linked respiratory process<ref>Maymó-Gatell, X., Anguish, T. and Zinder, S.H., 1999. Reductive dechlorination of chlorinated ethenes and 1, 2-dichloroethane by ''Dehalococcoides ethenogenes'' 195. Applied and Environmental Microbiology, 65(7), 3108-3113. [//www.enviro.wiki/images/2/22/Maymo-Gatell-1999-Reductive_dechlorination.pdf Report pdf]</ref><ref>Maymó-Gatell, X., Nijenhuis, I. and Zinder, S.H., 2001. Reductive dechlorination of cis-1,2-dichloroethene and vinyl chloride by ''Dehalococcoides ethenogenes''. Environmental Science & Technology, 35(3), 516-521. [https://doi.org/10.1021/es001285i doi: 10.1021/es001285i]</ref>. These cultures are able to reduce VC to ethene when growing with the other chlorinated ethenes as electron acceptors, but when provided with only VC, they are unable to grow. Under these circumstances, the transformation of VC to ethene is referred to as cometabolic, i.e., the transformation process is not linked to growth. In general, organohalide respiration occurs at a higher rate than reduction via cometabolism. Some microbes are capable of reducing VC to ethene via respiration, others are not. |
Organohalide respiration appears to be a widely-distributed process in nature, even in some environments that have not previously been contaminated by human activity<ref>Krzmarzick, M.J., Crary, B.B., Harding, J.J., Oyerinde, O.O., Leri, A.C., Myneni, S.C. and Novak, P.J., 2012. Natural niche for organohalide-respiring ''Chloroflexi''. Applied and Environmental Microbiology, 78(2), 393-401. [https://doi.org/10.1128/aem.06510-11 doi: 10.1128/AEM.06510-11]</ref>. The widespread distribution of organohalide respiring microbes is likely related to the natural formation of halogenated compounds, which have been generated on the planet long before human activity increased the rate and amount of halogenated compounds released to the environment. Having the capacity to dehalogenate is essential in natural systems in which organohalide compounds are also synthesized. | Organohalide respiration appears to be a widely-distributed process in nature, even in some environments that have not previously been contaminated by human activity<ref>Krzmarzick, M.J., Crary, B.B., Harding, J.J., Oyerinde, O.O., Leri, A.C., Myneni, S.C. and Novak, P.J., 2012. Natural niche for organohalide-respiring ''Chloroflexi''. Applied and Environmental Microbiology, 78(2), 393-401. [https://doi.org/10.1128/aem.06510-11 doi: 10.1128/AEM.06510-11]</ref>. The widespread distribution of organohalide respiring microbes is likely related to the natural formation of halogenated compounds, which have been generated on the planet long before human activity increased the rate and amount of halogenated compounds released to the environment. Having the capacity to dehalogenate is essential in natural systems in which organohalide compounds are also synthesized. | ||
| Line 97: | Line 101: | ||
The identification of dehalogenases has progressed to the point that quantification of key genes (e.g., ''tceA'', ''bvcA'', ''vcrA'', and others) in environmental samples is now a routine part of assessing the capacity for reductive dehalogenation to occur in the environment. | The identification of dehalogenases has progressed to the point that quantification of key genes (e.g., ''tceA'', ''bvcA'', ''vcrA'', and others) in environmental samples is now a routine part of assessing the capacity for reductive dehalogenation to occur in the environment. | ||
| − | [[File:Freedman Article 1 Figure 6.PNG|thumb|500 px|left|Figure 3. Schematic representation of a microbial cell carrying out organohalide respiration. Blue shape = the cell membrane; red oval = hydrogenase; yellow oval = electron carrier and proton translocation; orange oval = reductive dehalogenase; green shape = ATP synthase (modified from Jugder et al. (2016)<ref name = "Jugder2016"/>).]] | + | [[File:Freedman Article 1 Figure 6.PNG|thumb|500 px|left|Figure 3. Schematic representation of a microbial cell carrying out organohalide respiration. Blue shape = the cell membrane; red oval = hydrogenase; yellow oval = electron carrier and proton translocation; orange oval = reductive dehalogenase; green shape = ATP synthase (modified from Jugder et al. (2016)<ref name="Jugder2016" />).]] |
| − | Figure 3 presents a simplified schematic for how energy may be conserved during organohalide respiration. Many details of the process still need to be resolved and likely vary among the growing list of organohalide respiring microbes<ref name = "Jugder2016">Jugder, B.E., Ertan, H., Bohl, S., Lee, M., Marquis, C.P. and Manefield, M., 2016. Organohalide Respiring Bacteria and Reductive Dehalogenases: Key Tools in Organohalide Bioremediation. Frontiers in Microbiology, 7. [https://doi.org/10.3389/fmicb.2016.00249 doi: 10.3389/fmicb.2016.00249]</ref>. Reductive dehalogenases are a key component of the respiratory chain, which in the example shown culminates in development of a proton motive force and subsequent synthesis of adenosine triphosphate (ATP). | + | Figure 3 presents a simplified schematic for how energy may be conserved during organohalide respiration. Many details of the process still need to be resolved and likely vary among the growing list of organohalide respiring microbes<ref name="Jugder2016">Jugder, B.E., Ertan, H., Bohl, S., Lee, M., Marquis, C.P. and Manefield, M., 2016. Organohalide Respiring Bacteria and Reductive Dehalogenases: Key Tools in Organohalide Bioremediation. Frontiers in Microbiology, 7. [https://doi.org/10.3389/fmicb.2016.00249 doi: 10.3389/fmicb.2016.00249]</ref>. Reductive dehalogenases are a key component of the respiratory chain, which in the example shown culminates in development of a proton motive force and subsequent synthesis of adenosine triphosphate (ATP). |
| − | Among the various microbes capable of organohalide respiration, ''Dehalococcoides'' are the most frequently mentioned because of their capacity for complete reduction of chlorinated ethenes (i.e., PCE, TCE, DCEs, and VC) to ethene<ref>Löffler, F.E., Yan, J., Ritalahti, K.M., Adrian, L., Edwards, E.A., Konstantinidis, K.T., Müller, J.A., Fullerton, H., Zinder, S.H. and Spormann, A.M., 2013. Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. International Journal of Systematic and Evolutionary Microbiology, 63(2), 625-635. [https://doi.org/10.1099/ijs.0.034926-0 doi: 10.1099/ijs.0.034926-0]</ref>. At this point, members of this genus are the only known that are capable of completely dechlorinating the chlorinated ethenes to ethene, and more specifically, the steps from ''cis''-DCE to VC, and VC to ethene. Their versatility extends to use of many other organohalides as terminal electron acceptors, including polychlorinated biphenyls, chlorinated ethanes, and chlorinated benzenes. ''Dehalococcoides'' use only hydrogen as an electron donor and acetate as a carbon source. Their limited electron donor use stands in contrast to other organohalide respiring microbes, which are able to use a variety of organic compounds as electron donors. Other key types of organohalide respiring microbes (that cannot generate ethene) include ''Dehalobacter'', ''Dehalogenimonas'', ''Desulfitobacterium'', ''Sulfurospirillum'', and a number of ''Deltaproteobacteria''<ref name= "Adrian2016"/> 2. ''Dehalobacter'' includes microbes that respire chlorinated ethanes<ref>Sun, B., Griffin, B.M., Ayala-del-Rı́o, H.L., Hashsham, S.A. and Tiedje, J.M., 2002. Microbial dehalorespiration with 1, 1, 1-trichloroethane. Science, 298(5595), 1023-1025. [https://doi.org/10.1126/science.1074675 doi: 10.1126/science.1074675]</ref>, chlorinated ethenes<ref>Holliger, C., Hahn, D., Harmsen, H., Ludwig, W., Schumacher, W., Tindall, B., Vazquez, F., Weiss, N. and Zehnder, A.J., 1998. Dehalobacter restrictus gen. nov. and sp. nov., a strictly anaerobic bacterium that reductively dechlorinates tetra-and trichloroethene in an anaerobic respiration. Archives of Microbiology, 169(4), 313-321. [https://doi.org/10.1007/s002030050577 doi: 10.1007/s002030050577]</ref>, and chloroform<ref>Tang, S., Wang, P.H., Higgins, S.A., Löffler, F.E. and Edwards, E.A., 2016. Sister ''Dehalobacter'' Genomes reveal specialization in organohalide respiration and recent strain differentiation likely driven by chlorinated substrates. Frontiers in Microbiology, 7. [https://doi.org/10.3389/fmicb.2016.00100 doi: 10.3389/fmicb.2016.00100]</ref>. | + | Among the various microbes capable of organohalide respiration, ''Dehalococcoides'' are the most frequently mentioned because of their capacity for complete reduction of chlorinated ethenes (i.e., PCE, TCE, DCEs, and VC) to ethene<ref>Löffler, F.E., Yan, J., Ritalahti, K.M., Adrian, L., Edwards, E.A., Konstantinidis, K.T., Müller, J.A., Fullerton, H., Zinder, S.H. and Spormann, A.M., 2013. Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. International Journal of Systematic and Evolutionary Microbiology, 63(2), 625-635. [https://doi.org/10.1099/ijs.0.034926-0 doi: 10.1099/ijs.0.034926-0]</ref>. At this point, members of this genus are the only known that are capable of completely dechlorinating the chlorinated ethenes to ethene, and more specifically, the steps from ''cis''-DCE to VC, and VC to ethene. Their versatility extends to use of many other organohalides as terminal electron acceptors, including polychlorinated biphenyls, chlorinated ethanes, and chlorinated benzenes. ''Dehalococcoides'' use only hydrogen as an electron donor and acetate as a carbon source. Their limited electron donor use stands in contrast to other organohalide respiring microbes, which are able to use a variety of organic compounds as electron donors. Other key types of organohalide respiring microbes (that cannot generate ethene) include ''Dehalobacter'', ''Dehalogenimonas'', ''Desulfitobacterium'', ''Sulfurospirillum'', and a number of ''Deltaproteobacteria''<ref name="Adrian2016" /> 2. ''Dehalobacter'' includes microbes that respire chlorinated ethanes<ref>Sun, B., Griffin, B.M., Ayala-del-Rı́o, H.L., Hashsham, S.A. and Tiedje, J.M., 2002. Microbial dehalorespiration with 1, 1, 1-trichloroethane. Science, 298(5595), 1023-1025. [https://doi.org/10.1126/science.1074675 doi: 10.1126/science.1074675]</ref>, chlorinated ethenes<ref>Holliger, C., Hahn, D., Harmsen, H., Ludwig, W., Schumacher, W., Tindall, B., Vazquez, F., Weiss, N. and Zehnder, A.J., 1998. Dehalobacter restrictus gen. nov. and sp. nov., a strictly anaerobic bacterium that reductively dechlorinates tetra-and trichloroethene in an anaerobic respiration. Archives of Microbiology, 169(4), 313-321. [https://doi.org/10.1007/s002030050577 doi: 10.1007/s002030050577]</ref>, and chloroform<ref>Tang, S., Wang, P.H., Higgins, S.A., Löffler, F.E. and Edwards, E.A., 2016. Sister ''Dehalobacter'' Genomes reveal specialization in organohalide respiration and recent strain differentiation likely driven by chlorinated substrates. Frontiers in Microbiology, 7. [https://doi.org/10.3389/fmicb.2016.00100 doi: 10.3389/fmicb.2016.00100]</ref>. |
==Environmental Conditions== | ==Environmental Conditions== | ||
| − | With few exceptions, reductive dehalogenation occurs under anoxic conditions<ref name= "Adrian2016"/>. With the exception of nitrate, reductive dehalogenation has been observed in the presence of other anaerobic terminal electron acceptors, including ferric iron and sulfate. The effect of iron and sulfate on the rate and extent of reductive dechlorination is a matter of some debate, with some observing that these compounds (or the reduced forms) are inhibitory (e.g., via competition for hydrogen or the toxicity of sulfide) while others have shown that iron reduction is beneficial to the process<ref name= "Aulenta2006"/><ref>Wei, N. and Finneran, K.T., 2011. Influence of ferric iron on complete dechlorination of trichloroethylene (TCE) to ethene: Fe (III) reduction does not always inhibit complete dechlorination. Environmental Science & Technology, 45(17), 7422-7430. [https://doi.org/10.1021/es201501a doi 10.1021/es201501a]</ref>. An environment in which a community of anaerobes produces an excess of cobalamin (vitamin B<sub>12</sub>) is beneficial, since this coenzyme is an essential component of several dehalogenases<ref>Yan, J., Im, J., Yang, Y. and Löffler, F.E., 2013. Guided cobalamin biosynthesis supports ''Dehalococcoides mccartyi'' reductive dechlorination activity. Phil. Trans. R. Soc. B, 368(1616), 20120320. [https://doi.org/10.1098/rstb.2012.0320 doi: 10.1098/rstb.2012.0320]</ref>. Halogenated compounds can also be reduced in the presence of methanogens; however, methanogens compete for hydrogen as an electron donor and may at times limit the dechlorination reactions. | + | With few exceptions, reductive dehalogenation occurs under anoxic conditions<ref name="Adrian2016" />. With the exception of nitrate, reductive dehalogenation has been observed in the presence of other anaerobic terminal electron acceptors, including ferric iron and sulfate. The effect of iron and sulfate on the rate and extent of reductive dechlorination is a matter of some debate, with some observing that these compounds (or the reduced forms) are inhibitory (e.g., via competition for hydrogen or the toxicity of sulfide) while others have shown that iron reduction is beneficial to the process<ref name="Aulenta2006" /><ref>Wei, N. and Finneran, K.T., 2011. Influence of ferric iron on complete dechlorination of trichloroethylene (TCE) to ethene: Fe (III) reduction does not always inhibit complete dechlorination. Environmental Science & Technology, 45(17), 7422-7430. [https://doi.org/10.1021/es201501a doi 10.1021/es201501a]</ref>. An environment in which a community of anaerobes produces an excess of cobalamin (vitamin B<sub>12</sub>) is beneficial, since this coenzyme is an essential component of several dehalogenases<ref>Yan, J., Im, J., Yang, Y. and Löffler, F.E., 2013. Guided cobalamin biosynthesis supports ''Dehalococcoides mccartyi'' reductive dechlorination activity. Phil. Trans. R. Soc. B, 368(1616), 20120320. [https://doi.org/10.1098/rstb.2012.0320 doi: 10.1098/rstb.2012.0320]</ref>. Halogenated compounds can also be reduced in the presence of methanogens; however, methanogens compete for hydrogen as an electron donor and may at times limit the dechlorination reactions. |
Circumneutral pH is considered to be optimum for complete reduction of chlorinated ethenes to ethene<ref>Robinson, C., Barry, D.A., McCarty, P.L., Gerhard, J.I. and Kouznetsova, I, 2009. pH control for enhanced reductive bioremediation of chlorinated solvent source zones. Science of the Total Environment, 407(16), 4560-4573. [https://doi.org/10.1016/j.scitotenv.2009.03.029 doi 10.1016/j.scitotenv.2009.03.029]</ref><ref>Vainberg, S., Condee, C.W. and Steffan, R.J., 2009. Large-scale production of bacterial consortia for remediation of chlorinated solvent-contaminated groundwater. Journal of Industrial Microbiology & Biotechnology, 36(9), 1189-1197. [https://doi.org/10.1007/s10295-009-0600-5 doi: 10.1007/s10295-009-0600-5]</ref>. However, organohalide respiring microbes other than ''Dehalococcoides'' tolerate lower pH levels (e.g., as low as 4). There is growing evidence to indicate that strains of ''Dehalococcoides'' exist that are also tolerant of pH levels below circumneutral. For example, the pH range for a commonly used bioaugmentation culture that includes ''Dehalococcoides'' is 5.8-6.3 ([http://siremlab.com/kb-1-kb-1-plus/ SIREM]). This is an important consideration for bioaugmentation in low pH aquifers, since there are significant challenges associates with adjusting groundwater pH. | Circumneutral pH is considered to be optimum for complete reduction of chlorinated ethenes to ethene<ref>Robinson, C., Barry, D.A., McCarty, P.L., Gerhard, J.I. and Kouznetsova, I, 2009. pH control for enhanced reductive bioremediation of chlorinated solvent source zones. Science of the Total Environment, 407(16), 4560-4573. [https://doi.org/10.1016/j.scitotenv.2009.03.029 doi 10.1016/j.scitotenv.2009.03.029]</ref><ref>Vainberg, S., Condee, C.W. and Steffan, R.J., 2009. Large-scale production of bacterial consortia for remediation of chlorinated solvent-contaminated groundwater. Journal of Industrial Microbiology & Biotechnology, 36(9), 1189-1197. [https://doi.org/10.1007/s10295-009-0600-5 doi: 10.1007/s10295-009-0600-5]</ref>. However, organohalide respiring microbes other than ''Dehalococcoides'' tolerate lower pH levels (e.g., as low as 4). There is growing evidence to indicate that strains of ''Dehalococcoides'' exist that are also tolerant of pH levels below circumneutral. For example, the pH range for a commonly used bioaugmentation culture that includes ''Dehalococcoides'' is 5.8-6.3 ([http://siremlab.com/kb-1-kb-1-plus/ SIREM]). This is an important consideration for bioaugmentation in low pH aquifers, since there are significant challenges associates with adjusting groundwater pH. | ||
==Significance== | ==Significance== | ||
| − | Our understanding of the microbial processes that result in reductive removal of halogens from halogenated organic compounds has grown remarkably over the past three decades. The field has advanced from an assumption that halogenated organic compounds are non-biodegradable to our current understanding that not only do microbes perform dehalogenation reactions, but many do so via a growth-linked reductive, respiratory process (i.e. “breathing with chlorinated solvents”<ref name = "McCarty1997"/>). This underlying science has formed the basis for the practice of bioremediation, which has revolutionized the options available for cleaning up hazardous waste sites. Exciting new discoveries await that will open the door to biological treatment of emerging contaminants, as well as improvements in how to treat halogenated organics at complex sites where there are often mixtures of contaminants. | + | Our understanding of the microbial processes that result in reductive removal of halogens from halogenated organic compounds has grown remarkably over the past three decades. The field has advanced from an assumption that halogenated organic compounds are non-biodegradable to our current understanding that not only do microbes perform dehalogenation reactions, but many do so via a growth-linked reductive, respiratory process (i.e. “breathing with chlorinated solvents”<ref name="McCarty1997" />). This underlying science has formed the basis for the practice of bioremediation, which has revolutionized the options available for cleaning up hazardous waste sites. Exciting new discoveries await that will open the door to biological treatment of emerging contaminants, as well as improvements in how to treat halogenated organics at complex sites where there are often mixtures of contaminants. |
==References== | ==References== | ||
| − | <references/> | + | <references /> |
==See Also== | ==See Also== | ||
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1167 | + | |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1168 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1167 Aerobic and Anaerobic Transformation of cis-DCE and VC: Steps for Reliable Remediation] |
| + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1168 Characterization of the Aerobic Oxidation of cis-DCE and VC in Support of Bioremediation of Chloroethene-Contaminated Sites] | ||
*[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1169 Factors Affecting cis-DCE and VC Biological Transformation under Anaerobic Conditions] | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1169 Factors Affecting cis-DCE and VC Biological Transformation under Anaerobic Conditions] | ||
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1556 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1556 Characterization of Microbes Capable of Using Vinyl Chloride as a Sole Carbon and Energy Source by Anaerobic Oxidation] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1557 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1557 Elucidation of the Mechanisms and Environmental Relevance of cis-Dichloroethene and Vinyl Chloride Biodegradation] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1558 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-1558 Microbial Dichloroethene and Vinyl Chloride Oxidation and the Fate of Ethene and Ethane Under Anoxic Conditions] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-199921 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-199921 Push-Pull Tests for Evaluating the In-Situ Aerobic Treatment of Chlorinated Mixtures in Groundwater] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200516 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200516 Enhancing Natural Attenuation through Bioaugmentation with Aerobic Bacteria that Degrade cis-1,2-Dichloroethene] |
| − | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-201026/ER-201026 | + | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/Persistent-Contamination/ER-201026/ER-201026 Incorporating Aerobic Processes into Remedies for Large Chlorinated Solvent Plumes] |
*[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200316 Enhanced Oxidative Bioremediation of cis-Dichloroethene and Vinyl Chloride Using Electron Shuttles] | *[https://www.serdp-estcp.org/Program-Areas/Environmental-Restoration/Contaminated-Groundwater/ER-200316 Enhanced Oxidative Bioremediation of cis-Dichloroethene and Vinyl Chloride Using Electron Shuttles] | ||
| − | *[https://www.coursera.org/learn/natural-attenuation-of-groundwater-contaminants/lecture/6UJTE/biodegradation-mechanisms-chlorinated-solvents-vs-hydrocarbons | + | *[https://www.coursera.org/learn/natural-attenuation-of-groundwater-contaminants/lecture/6UJTE/biodegradation-mechanisms-chlorinated-solvents-vs-hydrocarbons Online Lecture Course - Chlorinated Solvents Biodegradation] |
Latest revision as of 21:35, 26 April 2022
Microbial removal of halogens from organic compounds by reductive processes forms the basis for many types of bioremediation technologies. The process was discovered within the last several decades and our understanding of how microbes perform this activity has improved significantly. Advances in our understanding of the microbiology of reductive dehalogenation have led to improvements in documenting natural attenuation and implementation of biostimulation and bioaugmentation. As a general rule, bioremediation is a lower cost approach to treatment of halogenated solvents than competing processes based on physical or chemical techniques (e.g., chemical oxidation). For practitioners, a working knowledge of microbial reductive processes is essential to successful application at hazardous waste sites.
Related Article(s):
Contributor(s): Dr. David L. Freedman
Key Resource(s):
- Enhanced Anaerobic Bioremediation of Chlorinated Solvents: Environmental Factors Influencing Microbial Activity and Their Relevance under Field Conditions[1].
- Organohalide Respiring Bacteria[2]
Introduction
Organic compounds with one or more halogens attached are referred to as halogenated organics. The halogens include chlorine (Cl), bromine (Br), fluorine (F), and iodine (I). For example, ethene (C2H4) is an organic compound. When three of the four hydrogen atoms on ethene are replaced with chlorine, the resulting compound is trichloroethene (TCE; C2HCl3).
The proliferation of halogenated organic compounds in the environment is a consequence of their widespread use in industrial activities. A critical part of many manufacturing processes involves removal of oil and grease from metal, fabrics, and other commodities. Because “like dissolves like,” a common way to remove oil and grease is to soak products in a nonpolar solvent. Non-halogenated hydrocarbons serve this purpose; however, accumulation of hydrocarbon vapors creates the risk of an explosion. Organic chemists solved this problem by adding halogens to the hydrocarbons, rendering them non-flammable. Use of halogenated organic compounds grew dramatically after World War II. With increased use came increased releases to the environment. Initially, halogenated solvents were thought to be inert in the environment and hence not to pose any risk to humans or wildlife. Gradually, the risks associated with chronic human exposure to halogenated solvents were revealed and concern grew about their fate in the environment. Most of the compounds on the United Nations list of persistent organic pollutants (first developed at the Stockholm Convention on Persistent Organic Pollutants) are halogenated organic compounds.
Dehalogenation
Microbes possess the ability to remove halogens from halogenated organic compounds. Dehalogenation may occur via a variety of reactions, including oxidation, reduction, or hydrolysis. The halogens are released as halides, i.e., Cl-, Br-, F-, and I-. The process of removing a halogen from a halogenated organic compound by a reductive reaction is referred to as reductive dehalogenation. Reductive reactions are ones in which electrons are transferred to the carbon-halogen bond, thereby lowering the oxidation state of the parent compound (example reactions below).
One of the earliest studies (1982) to demonstrate that microbes are capable of removing halogens from halogenated organic compounds used halobenzoates (e.g., 3-chlorobenzoate)[3]. Knowledge about microbial dehalogenation has since grown considerably and now forms the basis for bioremediation, a commonly applied strategy to treat halogenated organic compounds found in soils, groundwater, and wastewater.
Bioremediation
As a general rule, bioremediation is a lower cost approach to treatment of halogenated solvents than competing processes based on physical or chemical techniques (e.g., chemical oxidation). The discovery that microbes are capable of replacing the chlorines on tetrachloroethene (PCE) and TCE, the two most frequently encountered organic contaminants at hazardous waste sites, opened the door to development of current bioremediation strategies. Notably, there was a pause in interest when it was initially believed that the dechlorination process stopped at vinyl chloride (VC), which is more toxic than PCE, TCE, and dichloroethene (DCE) isomers. It was subsequently determined that microbial reduction of VC to ethene occurs[4]. Ethene is an acceptable endpoint since it poses no human health risks at the concentrations typically found in groundwater.
Although humans are responsible for a large influx of halogenated organics into the environment as a consequence of improper handling and disposal practices, it has also come to light that there are natural sources of halogenated organic compounds; nearly 5,000 have been catalogued to date[5]. For example, marine algae produce chloromethane as part of a chemical defense system to dissuade predation. Consequently, it is not too surprising that natural processes exist to break down halogenated organic compounds, and those processes have been harnessed to help clean up the excessive amounts released to the environment as a consequence of human activity.
There are several types of reaction pathways that involve reduction and dehalogenation, including hydrogenolysis and dihaloelimination[6]. Here, we detail each of these pathways and the conditions under which each is likely to be prevalent. It should be noted that many of the reactions are also possible via abiotic processes (e.g., via reaction with zerovalent iron (ZVI))[7]. The focus of this article is on biotic reductive processes.
Hydrogenolysis
Hydrogenolysis is the process by which a carbon—halogen bond is broken and hydrogen replaces the halogen substituent, resulting in release of a halide ion (R = organic compound, X = halide):
The process requires an input of reducing power, represented in the above equation by 2e-, or two electron equivalents. Hydrogenolysis is the most frequently observed reductive pathway, and as such it is commonly referred to as reductive dehalogenation. Nevertheless, other pathways are also reductive and result in dehalogenation, so the more correct description of the above reaction is hydrogenolysis. This descriptor derives in part from the fact that hydrogen (H2) is often a source of the electron equivalents:
Hydrogenolysis applies to any organohalide, yet it is most commonly associated with removal of chlorine from organic solvents and the term reductive dechlorination is often used synonymously (mostly amongst practitioners) to describe this reaction. As mentioned above, this is not quite correct, since other pathways are also reductive and result in removal of chlorine atoms, and the correct chemical mechanism is hydrogenolysis.
Hydrogenolysis applies to numerous categories of compounds; we highlight several of the major categories below. The process typically occurs via a respiratory process referred to as organohalide respiration (described below).
Chlorinated Ethenes
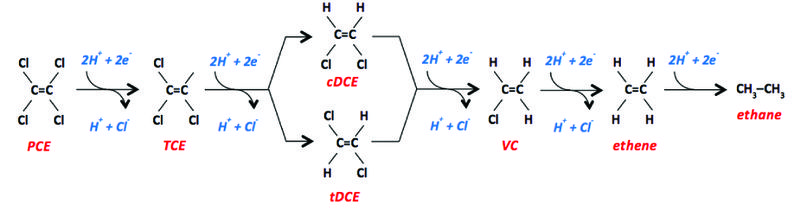
When applied to PCE, hydrogenolysis proceeds in 2e- steps through TCE, cis- or trans-1,2- DCE, VC, and ethene, which is also called ethylene (Fig. 1). cis-DCE is the more frequently identified of the 1,2-DCE isomers. Nevertheless, trans-DCE is predominant in some environments[8]. 1,1-DCE is another possible dichloroethene isomer, but it is not typically formed during microbial TCE respiration. The presence of 1,1-DCE in the environment is most typically associated with prior contamination by 1,1,1-trichloroethane, which undergoes several types of transformation, including the abiotic process of dehydrohalogenation to 1,1-DCE.
In some locations, further reduction of ethene to ethane has been reported. This is not a dechlorination reaction, but it is important to mention because ethane may be the terminal product from hydrogenolysis of chlorinated ethenes. Monitoring ethane is recommended for establishing a complete assessment of the fate of chlorinated ethenes.
The oxidation state of carbon in an organic compound varies from -4 (e.g., in CH4) to +4 (e.g., in CO2). The lower the oxidation state, the easier it is for oxidation to occur, and vice versa.
The oxidation state of the carbon in PCE is +4. With each successive reduction step (via an input of 2e-), the oxidation state of the carbon decreases by 2, so that the carbon in TCE has an oxidation state of +2, DCE has 0, VC has -2, and ethene has -4. For this category of contaminants, the final step is critical from a remediation perspective, since VC is a known human carcinogen while ethene and athnae pose no human health risks at the concentrations typically found in groundwater.
Chlorinated Ethanes
Like chlorinated ethenes, chlorinated ethanes are reduced by hydrogenolysis. One of the most widely evaluated compounds is 1,1,1-trichloroethane, which undergoes sequential reduction to 1,1-dichloroethane and chloroethane. Although further reduction to ethane is possible, it is a much slower reaction and chloroethane is typically regarded as the terminal product. This example serves to illustrate that hydrogenolysis does not always yield complete dechlorination.
Other commonly encountered chlorinated ethanes undergo hydrogenolysis, including reduction of 1,2-dichloroethane to chloroethane and 1,2-dichloropropane to 1- or 2-chloropropane.
Chlorinated Methanes
Carbon tetrachloride (tetrachloromethane) undergoes hydrogenolysis to chloroform (trichloromethane) and then methylene chloride (dichloromethane). These reactions are also catalyzed by reduced iron, which may be generated by iron-reducing bacteria.
Further reduction to chloromethane and methane is not commonly observed; other anaerobic biodegradation processes, such as organohalide fermentation, are more significant for dichloromethane and chloromethane.
Chlorinated Aromatic Compounds
Hydrogenolysis also occurs for chlorinated aromatic compounds, including chlorinated benzenes, polychlorinated biphenyls (PCBs), chlorinated dioxins (e.g., 2,3,7,8-tetrachlorodibenzo-p-dioxin, or TCDD) and furans, and polychlorinated phenols (e.g. pentachlorophenol, or PCP). For these compounds, the pathways are more complicated, since reduction proceeds through multiple isomers, depending on which position on the ring that each specific chlorine atom is removed. Like chlorinated ethanes and methanes, hydrogenolysis of chlorinated aromatic compounds is rarely complete, and the rate of reduction often decreases with a decreasing number of chlorine-carbon bonds. An important exception has been identification of cultures that reduce chlorinated benzenes completely to benzene[9][10].
Bromo- and Fluoro-Organic Compounds
Hydrogenolysis of brominated organic compounds has been documented. Examples include reduction of 1,2-dibromoethane (ethylene dibromide) to bromoethane and reduction of polybrominated diphenyl ethers. Likewise, defluorination also occurs. For example, reduction of vinyl fluoride to ethene has been reported. Nevertheless, reductive defluorination is characterized by slow rates, if it occurs at all. This is consistent with the general expectation that when the rate-limiting step for a reaction is cleavage of the carbon halogen bond, the order of reactivity is:
For example, hydrogenolysis of trichlorofluoromethane (CFC-11) typically results in accumulation of dichloro- and chlorofluoro-methane; further hydrogenolysis occurs at a much slower rate, if at all. Microbial reductive defluorination of perflurooctanoic acid (PFOA; used in the manufacture of Teflon) has not been reported to any appreciable extent[12].
Dihaloelimination
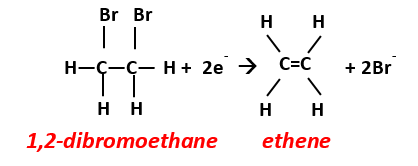
Dihaloelimination is the process by which two groups, e.g., a hydrogen and chlorine, are removed from adjacent carbon atoms, resulting in the formation of a double bond and release of two halide ions (X- = halide.):
Although less frequently encountered than hydrogenolysis, dihaloelimination is a critical pathway for several common groundwater contaminants, including reduction of 1,2-dichloroethane and 1,2-dibromoethane (more commonly referred to as ethylene dibromide, or EDB) to ethene[13]:
In this example, the oxidation state of the carbon decreases from -2 in 1,2-dibromoethane to -4 in ethene, i.e., by 2 electrons. Thus, the process is both reductive and results in removal of halides. Other examples of dihaloelimination include reduction of 1- or 2-chloropropane to propene.
Organohalide Respiration
A variety of microbes have developed pathways to conserve the energy made available when breaking carbon-halogen bonds via reduction (i.e., hydrogenolysis and dihaloelimination), by using the halogenated organic compounds as terminal electron acceptors[2]. This has led to the notion that certain microbes are capable of “breathing” halogenated organics, analogous to respiration involving oxygen as the electron acceptor[14]. When halogenated organic compounds serve as terminal electron acceptors, the process is referred to as organohalide respiration. The discovery of this process adds to the extensive list of terminal electron acceptors that microbes are capable of exploiting. Notably, a few types of microbes are obligate halorespirers, meaning the only known terminal electron acceptors for the cells are halogenated organic compounds. Other types of microbes are facultative with respect to their use of halogenated organics as terminal electron acceptors.
Under some circumstances, microbes carry out reductive dehalogenation but are unable to conserve energy from the process. For example, several strains of microbes are able to use PCE, TCE and cis-DCE as terminal electron acceptors, whereas reduction of VC to ethene is not a growth-linked respiratory process[15][16]. These cultures are able to reduce VC to ethene when growing with the other chlorinated ethenes as electron acceptors, but when provided with only VC, they are unable to grow. Under these circumstances, the transformation of VC to ethene is referred to as cometabolic, i.e., the transformation process is not linked to growth. In general, organohalide respiration occurs at a higher rate than reduction via cometabolism. Some microbes are capable of reducing VC to ethene via respiration, others are not.
Organohalide respiration appears to be a widely-distributed process in nature, even in some environments that have not previously been contaminated by human activity[17]. The widespread distribution of organohalide respiring microbes is likely related to the natural formation of halogenated compounds, which have been generated on the planet long before human activity increased the rate and amount of halogenated compounds released to the environment. Having the capacity to dehalogenate is essential in natural systems in which organohalide compounds are also synthesized.
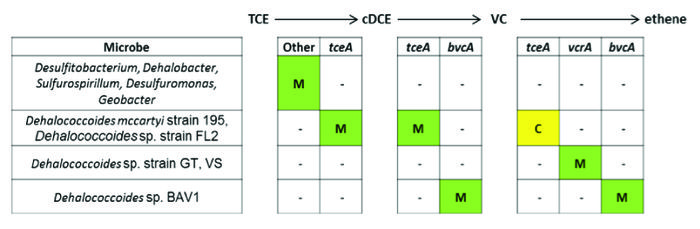
The process of organohalide respiration is centered on reductive dehalogenases, an iron–sulfur and coronoid containing family of enzymes that break carbon-halogen bonds. Among the best characterized genes are the ones involved in reductive dehalogenation of PCE, TCE, cis-DCE, and VC. Several microbes and enzymes are involved in each reduction step (Fig. 2).
The identification of dehalogenases has progressed to the point that quantification of key genes (e.g., tceA, bvcA, vcrA, and others) in environmental samples is now a routine part of assessing the capacity for reductive dehalogenation to occur in the environment.
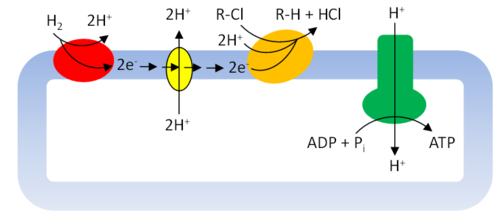
Figure 3 presents a simplified schematic for how energy may be conserved during organohalide respiration. Many details of the process still need to be resolved and likely vary among the growing list of organohalide respiring microbes[18]. Reductive dehalogenases are a key component of the respiratory chain, which in the example shown culminates in development of a proton motive force and subsequent synthesis of adenosine triphosphate (ATP).
Among the various microbes capable of organohalide respiration, Dehalococcoides are the most frequently mentioned because of their capacity for complete reduction of chlorinated ethenes (i.e., PCE, TCE, DCEs, and VC) to ethene[19]. At this point, members of this genus are the only known that are capable of completely dechlorinating the chlorinated ethenes to ethene, and more specifically, the steps from cis-DCE to VC, and VC to ethene. Their versatility extends to use of many other organohalides as terminal electron acceptors, including polychlorinated biphenyls, chlorinated ethanes, and chlorinated benzenes. Dehalococcoides use only hydrogen as an electron donor and acetate as a carbon source. Their limited electron donor use stands in contrast to other organohalide respiring microbes, which are able to use a variety of organic compounds as electron donors. Other key types of organohalide respiring microbes (that cannot generate ethene) include Dehalobacter, Dehalogenimonas, Desulfitobacterium, Sulfurospirillum, and a number of Deltaproteobacteria[2] 2. Dehalobacter includes microbes that respire chlorinated ethanes[20], chlorinated ethenes[21], and chloroform[22].
Environmental Conditions
With few exceptions, reductive dehalogenation occurs under anoxic conditions[2]. With the exception of nitrate, reductive dehalogenation has been observed in the presence of other anaerobic terminal electron acceptors, including ferric iron and sulfate. The effect of iron and sulfate on the rate and extent of reductive dechlorination is a matter of some debate, with some observing that these compounds (or the reduced forms) are inhibitory (e.g., via competition for hydrogen or the toxicity of sulfide) while others have shown that iron reduction is beneficial to the process[1][23]. An environment in which a community of anaerobes produces an excess of cobalamin (vitamin B12) is beneficial, since this coenzyme is an essential component of several dehalogenases[24]. Halogenated compounds can also be reduced in the presence of methanogens; however, methanogens compete for hydrogen as an electron donor and may at times limit the dechlorination reactions.
Circumneutral pH is considered to be optimum for complete reduction of chlorinated ethenes to ethene[25][26]. However, organohalide respiring microbes other than Dehalococcoides tolerate lower pH levels (e.g., as low as 4). There is growing evidence to indicate that strains of Dehalococcoides exist that are also tolerant of pH levels below circumneutral. For example, the pH range for a commonly used bioaugmentation culture that includes Dehalococcoides is 5.8-6.3 (SIREM). This is an important consideration for bioaugmentation in low pH aquifers, since there are significant challenges associates with adjusting groundwater pH.
Significance
Our understanding of the microbial processes that result in reductive removal of halogens from halogenated organic compounds has grown remarkably over the past three decades. The field has advanced from an assumption that halogenated organic compounds are non-biodegradable to our current understanding that not only do microbes perform dehalogenation reactions, but many do so via a growth-linked reductive, respiratory process (i.e. “breathing with chlorinated solvents”[14]). This underlying science has formed the basis for the practice of bioremediation, which has revolutionized the options available for cleaning up hazardous waste sites. Exciting new discoveries await that will open the door to biological treatment of emerging contaminants, as well as improvements in how to treat halogenated organics at complex sites where there are often mixtures of contaminants.
References
- ^ 1.0 1.1 Aulenta, F., Majone, M. and Tandoi, V., 2006. Enhanced anaerobic bioremediation of chlorinated solvents: environmental factors influencing microbial activity and their relevance under field conditions. Journal of Chemical Technology and Biotechnology, 81(9), 1463-1474. doi: 10.1002/jctb.1567
- ^ 2.0 2.1 2.2 2.3 Adrian, L. and Löffler, F., 2016. Organohalide Respiring Bacteria. 495 pgs. ISBN: 978-3-662-49873-6. doi: 10.1007/978-3-662-49875-0
- ^ Suflita, J.M., Horowitz, A., Shelton, D.R. and Tiedje, J.M., 1982. Dehalogenation: a novel pathway for the anaerobic biodegradation of haloaromatic compounds. Science, 218(4577), 1115-1117. doi: 10.1126/science.218.4577.1115
- ^ Freedman, D.L. and Gossett, J.M., 1989. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Applied and Environmental Microbiology, 55(9), 2144-2151. Report pdf
- ^ Gribble, G. W., 2010. Naturally Occurring Organohalogen Compounds - A Comprehensive Update. SpringerWien: New York. doi: 10.1007/978-3-211-99323-1
- ^ Vogel, T.M., Criddle, C.S. and McCarty, P.L., 1987. ES&T critical reviews: transformations of halogenated aliphatic compounds. Environmental Science & Technology, 21(8), 722-736. doi:10.1021/es00162a001
- ^ Arnold, W.A. and Roberts, A.L., 2000. Pathways and kinetics of chlorinated ethylene and chlorinated acetylene reaction with Fe(0) particles. Environmental Science & Technology, 34(9), 1794-1805. doi:10.1021/es990884q
- ^ Griffin, B.M., Tiedje, J.M. and Löffler, F.E., 2004. Anaerobic microbial reductive dechlorination of tetrachloroethene to predominately trans-1, 2-dichloroethene. Environmental Science & Technology, 38(16), 4300-4303. doi: 10.1021/es035439g
- ^ Fung, J.M., Weisenstein, B.P., Mack, E.E., Vidumsky, J.E., Ei, T.A. and Zinder, S.H., 2009. Reductive dehalogenation of dichlorobenzenes and monochlorobenzene to benzene in microcosms. Environmental Science & Technology, 43(7), 2302-2307. doi: 10.1021/es802131d
- ^ Nelson, J.L., Fung, J.M., Cadillo-Quiroz, H., Cheng, X. and Zinder, S.H., 2011. A role for Dehalobacter spp. in the reductive dehalogenation of dichlorobenzenes and monochlorobenzene. Environmental Science & Technology, 45(16), 6806-6813. doi: 10.1021/es200480k
- ^ Wackett, L.P., Logan, M.S., Blocki, F.A. and Bao-Li, C., 1992. A mechanistic perspective on bacterial metabolism of chlorinated methanes. Biodegradation, 3(1), 19-36. doi: 10.1007/BF00189633
- ^ Liou, J.C., Szostek, B., DeRito, C.M. and Madsen, E.L., 2010. Investigating the biodegradability of perfluorooctanoic acid. Chemosphere, 80(2), 176-183. doi: 10.1016/j.chemosphere.2010.03.009
- ^ Yu, R., Peethambaram, H.S., Falta, R.W., Verce, M.F., Henderson, J.K., Bagwell, C.E., Brigmon, R.L. and Freedman, D.L., 2013. Kinetics of 1,2-dichloroethane and 1,2-dibromoethane biodegradation in anaerobic enrichment cultures. Applied and Environmental Microbiology, 79(4), 1359-1367. doi: 10.1128/AEM.02163-12
- ^ 14.0 14.1 McCarty, P.L., 1997. Breathing with chlorinated solvents. Science, 276(5318), 1521-1522. doi: 10.1126/science.276.5318.1521
- ^ Maymó-Gatell, X., Anguish, T. and Zinder, S.H., 1999. Reductive dechlorination of chlorinated ethenes and 1, 2-dichloroethane by Dehalococcoides ethenogenes 195. Applied and Environmental Microbiology, 65(7), 3108-3113. Report pdf
- ^ Maymó-Gatell, X., Nijenhuis, I. and Zinder, S.H., 2001. Reductive dechlorination of cis-1,2-dichloroethene and vinyl chloride by Dehalococcoides ethenogenes. Environmental Science & Technology, 35(3), 516-521. doi: 10.1021/es001285i
- ^ Krzmarzick, M.J., Crary, B.B., Harding, J.J., Oyerinde, O.O., Leri, A.C., Myneni, S.C. and Novak, P.J., 2012. Natural niche for organohalide-respiring Chloroflexi. Applied and Environmental Microbiology, 78(2), 393-401. doi: 10.1128/AEM.06510-11
- ^ 18.0 18.1 Jugder, B.E., Ertan, H., Bohl, S., Lee, M., Marquis, C.P. and Manefield, M., 2016. Organohalide Respiring Bacteria and Reductive Dehalogenases: Key Tools in Organohalide Bioremediation. Frontiers in Microbiology, 7. doi: 10.3389/fmicb.2016.00249
- ^ Löffler, F.E., Yan, J., Ritalahti, K.M., Adrian, L., Edwards, E.A., Konstantinidis, K.T., Müller, J.A., Fullerton, H., Zinder, S.H. and Spormann, A.M., 2013. Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. International Journal of Systematic and Evolutionary Microbiology, 63(2), 625-635. doi: 10.1099/ijs.0.034926-0
- ^ Sun, B., Griffin, B.M., Ayala-del-Rı́o, H.L., Hashsham, S.A. and Tiedje, J.M., 2002. Microbial dehalorespiration with 1, 1, 1-trichloroethane. Science, 298(5595), 1023-1025. doi: 10.1126/science.1074675
- ^ Holliger, C., Hahn, D., Harmsen, H., Ludwig, W., Schumacher, W., Tindall, B., Vazquez, F., Weiss, N. and Zehnder, A.J., 1998. Dehalobacter restrictus gen. nov. and sp. nov., a strictly anaerobic bacterium that reductively dechlorinates tetra-and trichloroethene in an anaerobic respiration. Archives of Microbiology, 169(4), 313-321. doi: 10.1007/s002030050577
- ^ Tang, S., Wang, P.H., Higgins, S.A., Löffler, F.E. and Edwards, E.A., 2016. Sister Dehalobacter Genomes reveal specialization in organohalide respiration and recent strain differentiation likely driven by chlorinated substrates. Frontiers in Microbiology, 7. doi: 10.3389/fmicb.2016.00100
- ^ Wei, N. and Finneran, K.T., 2011. Influence of ferric iron on complete dechlorination of trichloroethylene (TCE) to ethene: Fe (III) reduction does not always inhibit complete dechlorination. Environmental Science & Technology, 45(17), 7422-7430. doi 10.1021/es201501a
- ^ Yan, J., Im, J., Yang, Y. and Löffler, F.E., 2013. Guided cobalamin biosynthesis supports Dehalococcoides mccartyi reductive dechlorination activity. Phil. Trans. R. Soc. B, 368(1616), 20120320. doi: 10.1098/rstb.2012.0320
- ^ Robinson, C., Barry, D.A., McCarty, P.L., Gerhard, J.I. and Kouznetsova, I, 2009. pH control for enhanced reductive bioremediation of chlorinated solvent source zones. Science of the Total Environment, 407(16), 4560-4573. doi 10.1016/j.scitotenv.2009.03.029
- ^ Vainberg, S., Condee, C.W. and Steffan, R.J., 2009. Large-scale production of bacterial consortia for remediation of chlorinated solvent-contaminated groundwater. Journal of Industrial Microbiology & Biotechnology, 36(9), 1189-1197. doi: 10.1007/s10295-009-0600-5
See Also
- Aerobic and Anaerobic Transformation of cis-DCE and VC: Steps for Reliable Remediation
- Characterization of the Aerobic Oxidation of cis-DCE and VC in Support of Bioremediation of Chloroethene-Contaminated Sites
- Factors Affecting cis-DCE and VC Biological Transformation under Anaerobic Conditions
- Characterization of Microbes Capable of Using Vinyl Chloride as a Sole Carbon and Energy Source by Anaerobic Oxidation
- Elucidation of the Mechanisms and Environmental Relevance of cis-Dichloroethene and Vinyl Chloride Biodegradation
- Microbial Dichloroethene and Vinyl Chloride Oxidation and the Fate of Ethene and Ethane Under Anoxic Conditions
- Push-Pull Tests for Evaluating the In-Situ Aerobic Treatment of Chlorinated Mixtures in Groundwater
- Enhancing Natural Attenuation through Bioaugmentation with Aerobic Bacteria that Degrade cis-1,2-Dichloroethene
- Incorporating Aerobic Processes into Remedies for Large Chlorinated Solvent Plumes
- Enhanced Oxidative Bioremediation of cis-Dichloroethene and Vinyl Chloride Using Electron Shuttles
- Online Lecture Course - Chlorinated Solvents Biodegradation